Spinal Fusion Therapy
Our bone growth therapy devices used in spinal applications are designed to enhance bone growth and the success rate of certain spinal fusions by stimulating the body’s own natural healing mechanism post-surgically. These noninvasive portable devices are intended to be used as part of a home treatment program prescribed by a healthcare professional.
Redefining Recovery for Spinal Fusion Therapy
The CervicalStim™ and SpinalStim™ devices provide safe and effective non-surgical treatment modalities to improve fusion healing. The devices use a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing. The SpinalStim and CervicalStim devices are anatomically designed, allowing for 360 degrees of PEMF treatment that can cover up to five vertebral levels at the fusion site.1-13

#1 Prescribed Bone Growth Stimulator14-15

Supported by the NASS coverage recommendations16

Over 1,000,000 prescribed devices

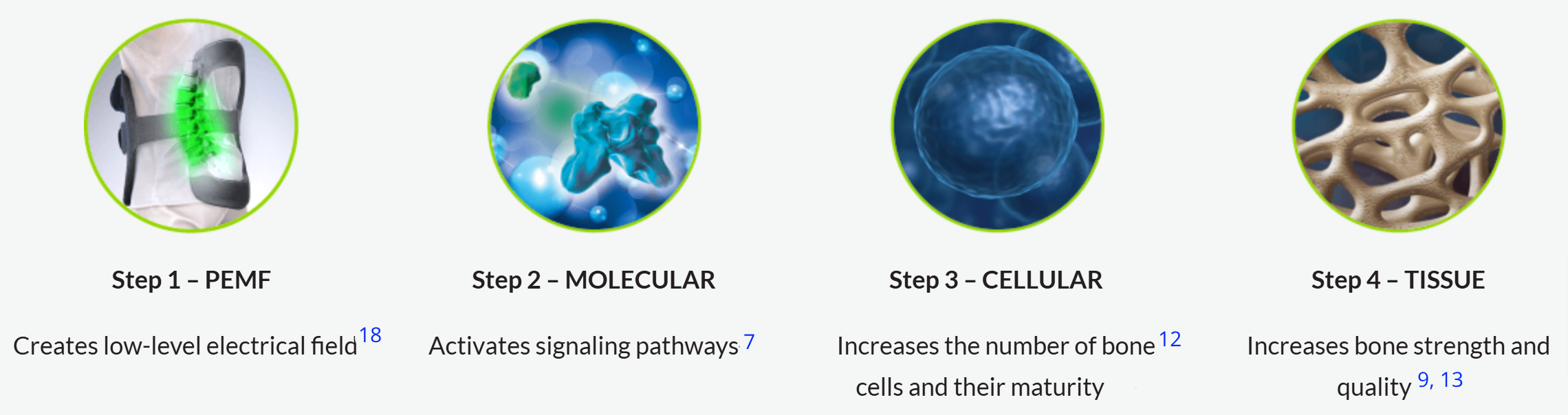
Pulsed Electromagnetic Field noninvasive spinal fusion therapy enhances bone growth and demonstrates statistical significance improves interbody fusion healing.
Why do Physicians Prescribe the SpinalStim Device?
The SpinalStim device has been approved by the FDA to be used after spinal fusion surgery or to be used to treat a failed fusion from previous surgery. For complete prescribing information, please refer to the Instruction Manual.
The SpinalStim device demonstrates statistical significance to improve interbody fusion healing.2,5
The ONLY bone growth stimulation therapy device approved by the FDA as both a lumbar spinal fusion adjunct and as a non-operative treatment for spinal pseudarthrosis.2,5,6
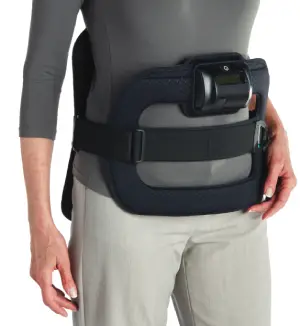




Pulsed Electromagnetic Field noninvasive cervical spinal fusion therapy enhances bone growth and increases the probability of fusion success.
Why do Physicians Prescribe the CervicalStim Device?
The Cervical Stim device has been approved by the FDA to be worn after cervical spine fusion surgery in patients at risk for non-fusion. Please refer to the instruction manual for complete prescribing information.

A significantly higher proportion of patients fused by 6 months when using the CervicalStim device compared to those without it.3-4
The ONLY bone growth stimulation device approved by the FDA to improve cervical fusion success.1,4



Devices Designed With the Patient in Mind
The SpinalStim™ and CervicalStim ™ devices are single pieces that are lightweight, flexible, and portable, allowing freedom of movement during treatment. An LCD and an audible alarm provide important feedback during treatment such as the operational status, treatment time remaining, battery capacity, and when paired with STIM onTrack™ patient mobile-app recovery is designed for success.
PEMF and the Healing Process
The result of these molecular, cellular and tissue processes is improved healing rates of a nonunion fracture or fusion.
Helping Physicians Monitor Their Patients' Recovery Remotely
Orthofix DIRECT™ and the STIM onTrack™ mobile application use advanced, innovative technology to connect physicians and patients when using bone healing solutions, regardless of where they are located.
Patient's Recovery Remotely
Easy to Navigate Features
Centralized Support for Patients Treating
With a Bone Growth Therapy DeviceTechnology Made With Trust
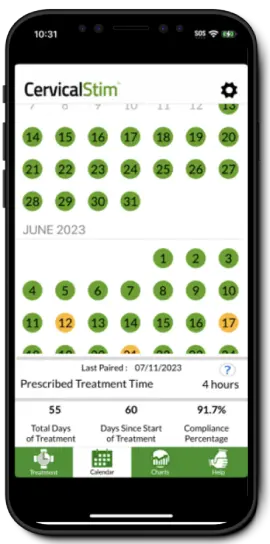


Easy to Navigate Features

Centralized Support for Patients Treating with a Bone Growth Therapy device
Brief Prescribing Information:
The CervicalStim™ device is indicated as an adjunct to cervical fusion surgery in patients at high risk for non-fusion; there are no known contraindications. Do not use this device if you have a cardiac pacemaker or defibrillator. Remove the device prior to any imaging procedures. Adverse effects may include increased pain, numbness and tingling, headache, migraines and nausea; these effects may or may not be directly related to use of the device.
The SpinalStim™ device is indicated as a spinal fusion adjunct to increase the probability of fusion success and as a nonoperative treatment of salvage of failed spinal fusion, where a minimum of nine months has elapsed since the last surgery. Cardiac pacemakers may be adversely affected by exposure to pulsed electromagnetic fields. Use of this device is contraindicated where the individual has an implanted cardiac pacemaker.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Care at 1-800-535-4492. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician
References:
- PMA P030034. December 2004
- PMA P850007/S6. February 1990
- Coric D, Bullard DE, Patel V, Ryaby J, Atkinson B, He D, Guyer R. Pulsed electromagnetic field stimulation may improve fusion rates in cervical arthrodesis in high-risk populations. Bone and Joint Res 2018; 7:124-130.
- Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8(3):436-44
- Mooney V. Pulsed electromagnetic fields: an adjunct to interbody spinal fusion surgery in the high risk patient. Surg Technol Int 1993, 2:405-410
- Simmons JW Jr, Mooney V, Thacker I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields. Am J Orthop. 2004;33(1):27-30
- Patterson TE, Sakai Y, Grabiner MD, et al. Exposure of murine cells to pulsed electromagnetic fields rapidly activates the mTOR-signaling pathway. Bioelectromagnetics. 2006;27(7):535-44
- Selvamurugan N, Kwok S, Vasilov A, Jefcoat SC, Partridge NC. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25(9):1213-20
- Midura RJ, Ibiwoye MO, Powell, KA, et al. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res. 2005;23:1035-46
- Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic
- Navarro, M., Michiardi, A., Castano, O., & Planell, J. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137-1158.
- Schnoke M, Midura RJ. Pulsed electromagnetic fields rapidly modulate intracellular signaling events in osteoblastic cells: comparison to parathyroid hormone and insulin. J Orthop Res. 2007;25(7):933-40
- Ibiwoye MO, Powell KA, Grabiner MD. Bone mass is preserved in a critical-sized osteotomy by low energy pulsed electromagnetic fields as quantitated by in vivo micro-computed tomography. J Orthop Res. 2004;22(5):1086-93
- iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP22_RPT), iData Research Inc (www.idataresearch.net) 2022
- iData Research Inc., U.S. Market for Orthopedic Trauma Devices (iDATA_USTRA22_RMS), iData Research Inc (www.idataresearch.net) 2022
- Spine.org
- Data on file 2023
- Bassett, CA. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit Rev Biomed Eng. 1989; 17(5):451-529
* VAS Neck Pain was recorded pre-operatively and post-operatively.
Orthofix products or services referenced herein are trademarks or registered trademarks of Orthofix Medical Inc. and its group of companies. All rights reserved